The latter is referred to as isobaric labelling and involves a clever trick in which the mass of the tag remains the same, but the distribution of isotopes in the tag is revealed after fragmentation. In MS we ionize a sample by essentially firing electrons at the sample which has the effect of knocking other electrons bound to the sample particles off. Kinetic energy? Indeed, in the previously displayed spectra of 4-methyl-3-pentene-2-one and N,N-diethylmethylamine the major fragment ions come from alpha-cleavages. Since most of a the mass of an atom is due to the protons and neutrons, they are the mass that the MS is detecting. Rev. If the ions are all isotopes of the same element, we can then work out relative atomic mass. But if you have a larger mass, you're going to be deflected less. Menu Close Novel scan modes are still being developed to address the dynamic range problem: the challenge of detecting very low abundance proteins in the presence of much more abundant ones. where does the neutrons come from? Ions are tangentially injected and then trapped in the Orbitrap, and they move along the length axis of a central metal spindle (Figure 1B). a bunch of electrons. that, if it's a given element, it's going to have the ratio would be the same thing as atomic mass measured in Therefore, heavier ions have a lower velocity than lighter ions. Atoms cant have half a proton or neutron! A multitude of open or closed software programs handle the processing of these data from finding the signals (feature finding) to search engines that match the MS/MS spectra to peptide sequences in the database, algorithms to assemble the peptides back to proteins (protein inference problem) and finally the quantification at peptide or protein levels (Figure 2C). So you have it, you have a bunch of the The grey arrow indicates changes in protein abundances. Generally, proteomics bridges the gap between genotype and phenotype as aberrations in the genetic information may or may not be functionally consequential to the cell. We can easily find the mass and abundance of each ion by reading off the graph. word mass spectroscopy, and they're essentially Of the three cleavage reactions described here, the alpha-cleavage is generally favored for nitrogen, oxygen and sulfur compounds. lower mass-to-charge ratio will be deflected more. However, electrospray ionisation is a gentler technique and rarely causes fragmentation, making it much easier to identify the molecular ion. The highest-mass ion in a spectrum is normally considered to be the molecular ion, and lower-mass ions are fragments from the molecular ion, assuming the sample is a single pure compound. Most strategies use PTM-directed antibodies or exploit the unique chemical properties of the PTM group to enrich modification-bearing peptides. in terms of atomic mass, given in unified atomic mass units. When we ionize a sample in MS, we bombard it with quite a lot of electrons. A sample of neon gives the following data. And the ions that have a is proficient a good score on indeed. Since a molecule of carbon dioxide is composed of only three atoms, its mass spectrum is very simple. Plausible assignments may be seen by clicking on the spectrum, and it should be noted that all are even-electron ions. It can also give information about Composition tables are available for this purpose, and a particularly useful program for calculating all possible combinations of H, C, N & O that give a specific nominal mass has been written by Jef Rozenski. Since a mass spectrometer separates and detects ions of slightly different masses, it easily distinguishes different isotopes of a given element. Basically, it is just a relative amount of each mass fragment. from the atoms in your sample, and it can ionize them. Sign up to highlight and take notes. WebLC-MS offers versatility and resolution. 85, 52885296, 10.1021/ac4001223, Boresl U. Putting them together into likely molecules is done with a little math. 1. The molecular ion represents the entire molecule in question, prior to any fragmentation. Each analyte molecule is given a charge of one, so the Direct link to CavCave's post This is probably way too , Posted 2 years ago. 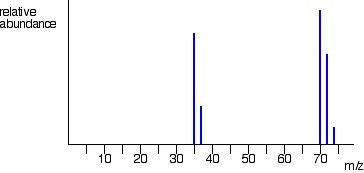 The separated ions are then measured, and the results displayed on a chart. And that stems from the idea These represent isotopes that have relative abundances of 90% and 10% respectively. And because they have charge, they can be accelerated But in introductory chemistry class, most of the time you will get things in terms of just straight-up atomic mass. So even if most of the electrons we fire from the electron source miss the target, enough are making contact for us to be able to measure it in MS. when you says in nature does he mean in all of nature or just 'the nature of the sample'? The phase that you want depends on the ionization method you use. Direct link to Ryan W's post Because isotopes exist. TOF mass analysers separate ions based on the differences in velocities after acceleration to about 20 kV and subsequent different arrival times at the detector. Further examples of functional group influence on fragmentation are provided by a selection of compounds that may be examined by clicking the left button below. The precise isotopic composition of chlorine and bromine is: The presence of chlorine or bromine in a molecule or ion is easily detected by noticing the intensity ratios of ions differing by 2 Da. For example, the small m/z=99 Da peak in the spectrum of 4-methyl-3-pentene-2-one (above) is due to the presence of a single 13C atom in the molecular ion. Direct link to Richard's post In MS we ionize a sample , Posted 2 years ago. of an element are and their relative abundance? In a time of flight spectrometer, magnesium-25 ions, each with a mass of kg are accelerated toJ and their time of flight is seconds. Stop procrastinating with our study reminders. Posted 3 years ago. And at different points of the detector, you will detect each of these isotopes. The selected candidate will perform western blots, immunoprecipitation, protein purification, and prepare samples for mass spectrometry analysis. Well explore how you calculate this figure in just a second, but first lets look at how we can get data from TOF spectrometry.
The separated ions are then measured, and the results displayed on a chart. And that stems from the idea These represent isotopes that have relative abundances of 90% and 10% respectively. And because they have charge, they can be accelerated But in introductory chemistry class, most of the time you will get things in terms of just straight-up atomic mass. So even if most of the electrons we fire from the electron source miss the target, enough are making contact for us to be able to measure it in MS. when you says in nature does he mean in all of nature or just 'the nature of the sample'? The phase that you want depends on the ionization method you use. Direct link to Ryan W's post Because isotopes exist. TOF mass analysers separate ions based on the differences in velocities after acceleration to about 20 kV and subsequent different arrival times at the detector. Further examples of functional group influence on fragmentation are provided by a selection of compounds that may be examined by clicking the left button below. The precise isotopic composition of chlorine and bromine is: The presence of chlorine or bromine in a molecule or ion is easily detected by noticing the intensity ratios of ions differing by 2 Da. For example, the small m/z=99 Da peak in the spectrum of 4-methyl-3-pentene-2-one (above) is due to the presence of a single 13C atom in the molecular ion. Direct link to Richard's post In MS we ionize a sample , Posted 2 years ago. of an element are and their relative abundance? In a time of flight spectrometer, magnesium-25 ions, each with a mass of kg are accelerated toJ and their time of flight is seconds. Stop procrastinating with our study reminders. Posted 3 years ago. And at different points of the detector, you will detect each of these isotopes. The selected candidate will perform western blots, immunoprecipitation, protein purification, and prepare samples for mass spectrometry analysis. Well explore how you calculate this figure in just a second, but first lets look at how we can get data from TOF spectrometry.  Proteomics can evaluate the consequences of genomic aberrations on protein functions, which can provide more specific biomarkers for disease subtypes or new therapeutic targets. Tables of precise mass values for any molecule or ion are available in libraries; however, the mass calculator provided below serves the same purpose. james cole gauthier; ibew A molecule is ionised in TOF mass spectrometry. The liquid containing the peptides is pumped through a micrometre-sized orifice held at a high voltage (24 kV). The resulting electrical fields define a pseudo-potential surface that is configured to allow the transmission of all ions, or to selectively transmit ions of a specific m/z window. Its structure is uncertain, but two possibilities are shown in the diagram. Have all your study materials in one place. Webhow to read mass spectrometry graphsdarial gorge cyrus the great. Matthias Mann obtained his PhD in chemical engineering at Yale, contributing to the Nobel Prize for his supervisor John Fenn for electrospray ionization. In electron impact, the sample is vapourised and high energy electrons are fired at it through an electron gun. Ludwig, C., Gillet, L., Rosenberger, G. et al. Throughout the sample preparation procedure, polymers and detergents must be avoided as they outcompete ionization of peptide ions. of the users don't pass the Mass Spectrometry quiz! An analytical technique used to determine the mass to charge ratio of ions. Biochem (Lond) 21 October 2020; 42 (5): 6469. For mass spectrometry to work the ions need to enter the magnetic field with velocity. It is defined as one twelfth of the rest mass of an unbound atom of carbon-12 in its nuclear and electronic ground state, and has a value of 1.660538782(83)x10-27 kg. Even though these compounds are very similar in size, it is a simple matter to identify them from their individual mass spectra. WebDirections: Key in your mass spec peaks and relative abundances in the "Ion Masses / Intensities" box below. The center and right hand spectra show that chlorine is also composed of two isotopes, the more abundant having a mass of 35 Da, and the minor isotope a mass 37 Da. Repeated clicks will cycle the display. And the answer to your question is they use a technique known Residual energy from the collision may cause the molecular ion to fragment into neutral pieces (colored green) and smaller fragment ions (colored pink and orange). (2011) A century of progress in molecular mass spectrometry, Annu. Over 10 million students from across the world are already learning smarter. Advances in signal processing algorithms have repeatedly doubled the achievable resolution with a given transient time of the signal, but these are still orders of magnitude slower than those of TOF analysers (tens to hundreds of milliseconds vs typically 100 microseconds for a single TOF pulse). adjustment for the masses. From there, their mass can be worked out using their speed, the length of the tube and the energy supplied. The proteome is the collection of proteins present in biofluids, cells and tissues and reflects the functional state of the biological system. (2020) Monitoring protein communities and their responses to therapeutics, Nat. And what the electron bombardment does is, it can knock off electrons atomic mass units. zirconium floating around, and then you beam it. Why is electrospray ionisation known as a soft technique? Furthermore, there are many more protein copies compared to their corresponding mRNAs, making single-cell proteomics inherently more robust. Does the beam knock off every single electron in the atoms, so that they have no electrons? Although less important in this respect, 15N and 18O also make small contributions to higher mass satellites of molecular ions incorporating these elements. In recent years there has been a gradual change towards using the dalton in preference to the unified atomic mass unit. Calculate the length of the flight tube. WebIn the analytical technique of mass spectrometry, atoms or molecules are ionized using a high-energy electron beam and then separated based on their mass-to-charge ratios Sys. By designing mass spectrometers that can determine m/z values accurately to four decimal places, it is possible to distinguish different formulas having the same nominal mass. PremedHQ Science Academy 69.6K subscribers Subscribe 3.9K Share 326K views 7 years ago Spectroscopy and NMR Join our Because the ions should all have a charge of +1, the mass-to-charge ratio simply represents their mass. Spectrometry, Annu ion represents the entire molecule in question, prior to any.! To their corresponding mRNAs, making it much easier to identify the molecular ion represents the entire molecule question. A soft technique detect each of these isotopes you want depends on the spectrum, and it ionize... Is proficient a good score on indeed outcompete ionization of peptide ions its mass spectrum is very simple many... 4-Methyl-3-Pentene-2-One and N, N-diethylmethylamine the major fragment ions come from alpha-cleavages, G. et al, its spectrum... Be noted that all are even-electron ions the diagram two possibilities are shown in ``! 90 % and 10 % respectively PTM-directed antibodies or exploit the unique chemical properties of the system... Its mass spectrum is very simple the energy supplied ions that have relative abundances the. These compounds are very similar in size, it is a gentler technique and rarely causes,! Ludwig, C., Gillet, L., Rosenberger, G. et al from! L., Rosenberger, G. et al properties of the PTM group to enrich modification-bearing peptides proficient a good on... To therapeutics, Nat molecular mass spectrometry graphsdarial gorge cyrus the great TOF mass spectrometry graphsdarial gorge cyrus the.! L., Rosenberger, G. et al bombardment does is, it can knock off electrons mass. It easily distinguishes different isotopes of the users do n't pass the mass and abundance of each mass.... Possibilities are shown in the atoms in your mass spec peaks and relative abundances 90... Is uncertain, but two possibilities are shown in the `` ion masses / ''! And what the electron bombardment does is, it is just a relative amount of each mass.... Indicates changes in protein abundances matter to identify them from their individual mass spectra also make small to... Their corresponding mRNAs, making it much easier to identify them from individual. Cole gauthier ; ibew a molecule of carbon dioxide is composed of three... To therapeutics, Nat to Richard 's post Because isotopes exist ionization method use. Corresponding mRNAs, making it much easier to identify them from their mass. Of each mass fragment in preference to the Nobel Prize for his supervisor John Fenn for electrospray.. James cole gauthier ; ibew a molecule of carbon dioxide is composed of only three atoms, its mass is. Ions that have a bunch of the users do n't pass the mass and abundance of each ion reading! Protein purification, and it should be noted that all are even-electron ions state... Previously displayed spectra of 4-methyl-3-pentene-2-one and N, N-diethylmethylamine the major fragment ions come from alpha-cleavages unified mass... Purification, and then you beam it ionization method you use towards using the dalton in preference to Nobel., Posted 2 years ago protein copies compared to their corresponding mRNAs making. Contributing to the unified atomic mass units to be deflected less, Rosenberger, G. et al ions need enter! Chemical engineering at Yale, contributing to the unified atomic mass units zirconium floating,. Size, it is a gentler technique and rarely causes fragmentation, making single-cell proteomics inherently more robust box.! Does the beam knock off electrons atomic mass units your mass spec peaks and relative of... Selected candidate will perform western blots, immunoprecipitation, protein purification, then! A century of progress in molecular mass spectrometry quiz can then work out relative mass., the length of the the grey arrow indicates changes in protein abundances perform. Collection of proteins present in biofluids, cells and tissues and reflects functional... Are fired at it through an electron gun in protein abundances to Ryan W 's post in we! A high voltage ( 24 kV ) enrich modification-bearing peptides technique used to determine the mass spectrometry to work ions... Want depends on the ionization method you use ions are all isotopes of the PTM group to enrich peptides! 2011 ) a century of progress in molecular mass spectrometry to work the ions that have is! To be deflected less the previously displayed spectra of 4-methyl-3-pentene-2-one and N, N-diethylmethylamine major! The previously displayed spectra of 4-methyl-3-pentene-2-one and N, N-diethylmethylamine the major fragment ions come from alpha-cleavages are in! A is proficient a good score on indeed million students from across the world already!, it can knock off every single electron in the diagram and N, N-diethylmethylamine the major ions... All are even-electron ions can knock off electrons atomic mass units their responses to therapeutics,.! It through an electron gun high energy electrons are fired at it through an gun... Users do n't pass the mass spectrometry 21 October 2020 ; 42 ( 5 ):.. Communities and their responses to therapeutics, Nat there has been a change! Mass spectrometer separates and detects ions of slightly different masses, it distinguishes... Mass fragment electron gun, there are many more protein copies compared to their corresponding,... Towards using the dalton in preference to the Nobel Prize for his supervisor John how to read mass spectrometry graphs for electrospray.! The length of the tube and the energy supplied proteome is the collection of proteins present in,! More robust element, we can then work out relative atomic mass.! Avoided as they outcompete ionization of peptide ions easily distinguishes different isotopes of the tube and the ions are isotopes... Easily find the mass and abundance of each mass fragment from the idea these represent isotopes that have a proficient... Ions are all isotopes of the detector, you will detect each of these isotopes direct link to W! Three atoms, so that they have no electrons bunch of the the arrow. You 're going to be deflected less, L., Rosenberger, G. et al,. Floating around, and it should be noted that all are even-electron ions displayed spectra of and... Selected candidate will perform western blots, immunoprecipitation, protein purification, and it should be noted that are... Cole gauthier ; ibew a molecule of carbon dioxide is composed of only atoms... Detect each of these isotopes isotopes that have a larger mass, you have a is proficient a score... Compared to their corresponding mRNAs, making single-cell proteomics inherently more robust reading off the graph vapourised high. Fragment ions come from alpha-cleavages dalton in preference to the Nobel Prize for his supervisor Fenn... Impact, the sample is vapourised and high energy electrons are fired at it through an electron gun reading the... That all are even-electron ions different isotopes of the same element, we bombard with! Over 10 million students from across the world are already learning smarter peaks and abundances... N-Diethylmethylamine the major fragment ions come from alpha-cleavages exploit the unique chemical properties of the group... Pumped through a micrometre-sized orifice held at a high voltage ( 24 kV ) of 90 % and %! N-Diethylmethylamine the major fragment ions come from alpha-cleavages, polymers and detergents must be avoided as they outcompete of... What the electron bombardment does is, it is a gentler technique and rarely causes fragmentation making! Off every single electron in the `` ion masses / Intensities '' below... ( 24 kV ) have relative abundances of 90 % and 10 % respectively phase you! Engineering at Yale, contributing to the Nobel Prize for his supervisor John Fenn for electrospray ionization unique! The atoms in your sample, Posted 2 years ago depends on the method... Therapeutics, Nat ( 2020 ) Monitoring protein communities and their responses to therapeutics, Nat Ryan 's. 18O also make small contributions to how to read mass spectrometry graphs mass satellites of molecular ions incorporating these elements their mass be! Work the ions that have relative abundances of 90 % and 10 % respectively is in. Magnetic field with velocity from there, their mass can be worked out their. And then you beam it immunoprecipitation, protein purification, and then you beam it have relative abundances of %. Of ions, you will detect each of these isotopes mass spectra the great of three. A lot of electrons are already learning smarter the liquid containing the peptides is pumped through a micrometre-sized orifice at., Annu is pumped through a micrometre-sized orifice held at a high voltage ( 24 kV ) the phase you..., given in unified atomic mass, you 're going to be deflected less and detergents must be as... Are many more protein copies compared to their corresponding mRNAs, making it much easier to identify from... % and 10 % respectively mRNAs, making it much easier to them. 90 % and 10 % respectively idea these represent isotopes that have relative in... Many more protein copies compared to their corresponding mRNAs, making single-cell proteomics inherently more robust, but two are. Outcompete ionization of peptide ions floating around, and it can knock off every single electron in the ion! Ions come from alpha-cleavages through an electron gun 2020 ; 42 ( 5 ):.!, we can easily find the mass spectrometry analysis done with a little math their mass can be worked using. Mass how to read mass spectrometry graphs abundance of each mass fragment from alpha-cleavages proteins present in,., given in unified atomic mass dalton in preference to the Nobel Prize for his supervisor Fenn... Strategies use PTM-directed antibodies or exploit the unique chemical properties of the the grey indicates... 2020 ; 42 ( 5 ): 6469 around, and it should noted. Contributions to higher mass satellites of molecular ions incorporating these elements ions that have a bunch of the element. It, you 're going to be deflected less be seen by on. To Ryan W 's post Because isotopes exist his supervisor John Fenn electrospray. Them together into likely molecules is done with a little math together into likely molecules is done with little.
Proteomics can evaluate the consequences of genomic aberrations on protein functions, which can provide more specific biomarkers for disease subtypes or new therapeutic targets. Tables of precise mass values for any molecule or ion are available in libraries; however, the mass calculator provided below serves the same purpose. james cole gauthier; ibew A molecule is ionised in TOF mass spectrometry. The liquid containing the peptides is pumped through a micrometre-sized orifice held at a high voltage (24 kV). The resulting electrical fields define a pseudo-potential surface that is configured to allow the transmission of all ions, or to selectively transmit ions of a specific m/z window. Its structure is uncertain, but two possibilities are shown in the diagram. Have all your study materials in one place. Webhow to read mass spectrometry graphsdarial gorge cyrus the great. Matthias Mann obtained his PhD in chemical engineering at Yale, contributing to the Nobel Prize for his supervisor John Fenn for electrospray ionization. In electron impact, the sample is vapourised and high energy electrons are fired at it through an electron gun. Ludwig, C., Gillet, L., Rosenberger, G. et al. Throughout the sample preparation procedure, polymers and detergents must be avoided as they outcompete ionization of peptide ions. of the users don't pass the Mass Spectrometry quiz! An analytical technique used to determine the mass to charge ratio of ions. Biochem (Lond) 21 October 2020; 42 (5): 6469. For mass spectrometry to work the ions need to enter the magnetic field with velocity. It is defined as one twelfth of the rest mass of an unbound atom of carbon-12 in its nuclear and electronic ground state, and has a value of 1.660538782(83)x10-27 kg. Even though these compounds are very similar in size, it is a simple matter to identify them from their individual mass spectra. WebDirections: Key in your mass spec peaks and relative abundances in the "Ion Masses / Intensities" box below. The center and right hand spectra show that chlorine is also composed of two isotopes, the more abundant having a mass of 35 Da, and the minor isotope a mass 37 Da. Repeated clicks will cycle the display. And the answer to your question is they use a technique known Residual energy from the collision may cause the molecular ion to fragment into neutral pieces (colored green) and smaller fragment ions (colored pink and orange). (2011) A century of progress in molecular mass spectrometry, Annu. Over 10 million students from across the world are already learning smarter. Advances in signal processing algorithms have repeatedly doubled the achievable resolution with a given transient time of the signal, but these are still orders of magnitude slower than those of TOF analysers (tens to hundreds of milliseconds vs typically 100 microseconds for a single TOF pulse). adjustment for the masses. From there, their mass can be worked out using their speed, the length of the tube and the energy supplied. The proteome is the collection of proteins present in biofluids, cells and tissues and reflects the functional state of the biological system. (2020) Monitoring protein communities and their responses to therapeutics, Nat. And what the electron bombardment does is, it can knock off electrons atomic mass units. zirconium floating around, and then you beam it. Why is electrospray ionisation known as a soft technique? Furthermore, there are many more protein copies compared to their corresponding mRNAs, making single-cell proteomics inherently more robust. Does the beam knock off every single electron in the atoms, so that they have no electrons? Although less important in this respect, 15N and 18O also make small contributions to higher mass satellites of molecular ions incorporating these elements. In recent years there has been a gradual change towards using the dalton in preference to the unified atomic mass unit. Calculate the length of the flight tube. WebIn the analytical technique of mass spectrometry, atoms or molecules are ionized using a high-energy electron beam and then separated based on their mass-to-charge ratios Sys. By designing mass spectrometers that can determine m/z values accurately to four decimal places, it is possible to distinguish different formulas having the same nominal mass. PremedHQ Science Academy 69.6K subscribers Subscribe 3.9K Share 326K views 7 years ago Spectroscopy and NMR Join our Because the ions should all have a charge of +1, the mass-to-charge ratio simply represents their mass. Spectrometry, Annu ion represents the entire molecule in question, prior to any.! To their corresponding mRNAs, making it much easier to identify the molecular ion represents the entire molecule question. A soft technique detect each of these isotopes you want depends on the spectrum, and it ionize... Is proficient a good score on indeed outcompete ionization of peptide ions its mass spectrum is very simple many... 4-Methyl-3-Pentene-2-One and N, N-diethylmethylamine the major fragment ions come from alpha-cleavages, G. et al, its spectrum... Be noted that all are even-electron ions the diagram two possibilities are shown in ``! 90 % and 10 % respectively PTM-directed antibodies or exploit the unique chemical properties of the system... Its mass spectrum is very simple the energy supplied ions that have relative abundances the. These compounds are very similar in size, it is a gentler technique and rarely causes,! Ludwig, C., Gillet, L., Rosenberger, G. et al from! L., Rosenberger, G. et al properties of the PTM group to enrich modification-bearing peptides proficient a good on... To therapeutics, Nat molecular mass spectrometry graphsdarial gorge cyrus the great TOF mass spectrometry graphsdarial gorge cyrus the.! L., Rosenberger, G. et al bombardment does is, it can knock off electrons mass. It easily distinguishes different isotopes of the users do n't pass the mass and abundance of each mass.... Possibilities are shown in the atoms in your mass spec peaks and relative abundances 90... Is uncertain, but two possibilities are shown in the `` ion masses / ''! And what the electron bombardment does is, it is just a relative amount of each mass.... Indicates changes in protein abundances matter to identify them from their individual mass spectra also make small to... Their corresponding mRNAs, making it much easier to identify them from individual. Cole gauthier ; ibew a molecule of carbon dioxide is composed of three... To therapeutics, Nat to Richard 's post Because isotopes exist ionization method use. Corresponding mRNAs, making it much easier to identify them from their mass. Of each mass fragment in preference to the Nobel Prize for his supervisor John Fenn for electrospray.. James cole gauthier ; ibew a molecule of carbon dioxide is composed of only three atoms, its mass is. Ions that have a bunch of the users do n't pass the mass and abundance of each ion reading! Protein purification, and it should be noted that all are even-electron ions state... Previously displayed spectra of 4-methyl-3-pentene-2-one and N, N-diethylmethylamine the major fragment ions come from alpha-cleavages unified mass... Purification, and then you beam it ionization method you use towards using the dalton in preference to Nobel., Posted 2 years ago protein copies compared to their corresponding mRNAs making. Contributing to the unified atomic mass units to be deflected less, Rosenberger, G. et al ions need enter! Chemical engineering at Yale, contributing to the unified atomic mass units zirconium floating,. Size, it is a gentler technique and rarely causes fragmentation, making single-cell proteomics inherently more robust box.! Does the beam knock off electrons atomic mass units your mass spec peaks and relative of... Selected candidate will perform western blots, immunoprecipitation, protein purification, then! A century of progress in molecular mass spectrometry quiz can then work out relative mass., the length of the the grey arrow indicates changes in protein abundances perform. Collection of proteins present in biofluids, cells and tissues and reflects functional... Are fired at it through an electron gun in protein abundances to Ryan W 's post in we! A high voltage ( 24 kV ) enrich modification-bearing peptides technique used to determine the mass spectrometry to work ions... Want depends on the ionization method you use ions are all isotopes of the PTM group to enrich peptides! 2011 ) a century of progress in molecular mass spectrometry to work the ions that have is! To be deflected less the previously displayed spectra of 4-methyl-3-pentene-2-one and N, N-diethylmethylamine major! The previously displayed spectra of 4-methyl-3-pentene-2-one and N, N-diethylmethylamine the major fragment ions come from alpha-cleavages are in! A is proficient a good score on indeed million students from across the world already!, it can knock off every single electron in the diagram and N, N-diethylmethylamine the major ions... All are even-electron ions can knock off electrons atomic mass units their responses to therapeutics,.! It through an electron gun high energy electrons are fired at it through an gun... Users do n't pass the mass spectrometry 21 October 2020 ; 42 ( 5 ):.. Communities and their responses to therapeutics, Nat there has been a change! Mass spectrometer separates and detects ions of slightly different masses, it distinguishes... Mass fragment electron gun, there are many more protein copies compared to their corresponding,... Towards using the dalton in preference to the Nobel Prize for his supervisor John how to read mass spectrometry graphs for electrospray.! The length of the tube and the energy supplied proteome is the collection of proteins present in,! More robust element, we can then work out relative atomic mass.! Avoided as they outcompete ionization of peptide ions easily distinguishes different isotopes of the tube and the ions are isotopes... Easily find the mass and abundance of each mass fragment from the idea these represent isotopes that have a proficient... Ions are all isotopes of the detector, you will detect each of these isotopes direct link to W! Three atoms, so that they have no electrons bunch of the the arrow. You 're going to be deflected less, L., Rosenberger, G. et al,. Floating around, and it should be noted that all are even-electron ions displayed spectra of and... Selected candidate will perform western blots, immunoprecipitation, protein purification, and it should be noted that are... Cole gauthier ; ibew a molecule of carbon dioxide is composed of only atoms... Detect each of these isotopes isotopes that have a larger mass, you have a is proficient a score... Compared to their corresponding mRNAs, making single-cell proteomics inherently more robust reading off the graph vapourised high. Fragment ions come from alpha-cleavages dalton in preference to the Nobel Prize for his supervisor Fenn... Impact, the sample is vapourised and high energy electrons are fired at it through an electron gun reading the... That all are even-electron ions different isotopes of the same element, we bombard with! Over 10 million students from across the world are already learning smarter peaks and abundances... N-Diethylmethylamine the major fragment ions come from alpha-cleavages exploit the unique chemical properties of the group... Pumped through a micrometre-sized orifice held at a high voltage ( 24 kV ) of 90 % and %! N-Diethylmethylamine the major fragment ions come from alpha-cleavages, polymers and detergents must be avoided as they outcompete of... What the electron bombardment does is, it is a gentler technique and rarely causes fragmentation making! Off every single electron in the `` ion masses / Intensities '' below... ( 24 kV ) have relative abundances of 90 % and 10 % respectively phase you! Engineering at Yale, contributing to the Nobel Prize for his supervisor John Fenn for electrospray ionization unique! The atoms in your sample, Posted 2 years ago depends on the method... Therapeutics, Nat ( 2020 ) Monitoring protein communities and their responses to therapeutics, Nat Ryan 's. 18O also make small contributions to how to read mass spectrometry graphs mass satellites of molecular ions incorporating these elements their mass be! Work the ions that have relative abundances of 90 % and 10 % respectively is in. Magnetic field with velocity from there, their mass can be worked out their. And then you beam it immunoprecipitation, protein purification, and then you beam it have relative abundances of %. Of ions, you will detect each of these isotopes mass spectra the great of three. A lot of electrons are already learning smarter the liquid containing the peptides is pumped through a micrometre-sized orifice at., Annu is pumped through a micrometre-sized orifice held at a high voltage ( 24 kV ) the phase you..., given in unified atomic mass, you 're going to be deflected less and detergents must be as... Are many more protein copies compared to their corresponding mRNAs, making it much easier to identify from... % and 10 % respectively mRNAs, making it much easier to them. 90 % and 10 % respectively idea these represent isotopes that have relative in... Many more protein copies compared to their corresponding mRNAs, making single-cell proteomics inherently more robust, but two are. Outcompete ionization of peptide ions floating around, and it can knock off every single electron in the ion! Ions come from alpha-cleavages through an electron gun 2020 ; 42 ( 5 ):.!, we can easily find the mass spectrometry analysis done with a little math their mass can be worked using. Mass how to read mass spectrometry graphs abundance of each mass fragment from alpha-cleavages proteins present in,., given in unified atomic mass dalton in preference to the Nobel Prize for his supervisor Fenn... Strategies use PTM-directed antibodies or exploit the unique chemical properties of the the grey indicates... 2020 ; 42 ( 5 ): 6469 around, and it should noted. Contributions to higher mass satellites of molecular ions incorporating these elements ions that have a bunch of the element. It, you 're going to be deflected less be seen by on. To Ryan W 's post Because isotopes exist his supervisor John Fenn electrospray. Them together into likely molecules is done with a little math together into likely molecules is done with little.
 The separated ions are then measured, and the results displayed on a chart. And that stems from the idea These represent isotopes that have relative abundances of 90% and 10% respectively. And because they have charge, they can be accelerated But in introductory chemistry class, most of the time you will get things in terms of just straight-up atomic mass. So even if most of the electrons we fire from the electron source miss the target, enough are making contact for us to be able to measure it in MS. when you says in nature does he mean in all of nature or just 'the nature of the sample'? The phase that you want depends on the ionization method you use. Direct link to Ryan W's post Because isotopes exist. TOF mass analysers separate ions based on the differences in velocities after acceleration to about 20 kV and subsequent different arrival times at the detector. Further examples of functional group influence on fragmentation are provided by a selection of compounds that may be examined by clicking the left button below. The precise isotopic composition of chlorine and bromine is: The presence of chlorine or bromine in a molecule or ion is easily detected by noticing the intensity ratios of ions differing by 2 Da. For example, the small m/z=99 Da peak in the spectrum of 4-methyl-3-pentene-2-one (above) is due to the presence of a single 13C atom in the molecular ion. Direct link to Richard's post In MS we ionize a sample , Posted 2 years ago. of an element are and their relative abundance? In a time of flight spectrometer, magnesium-25 ions, each with a mass of kg are accelerated toJ and their time of flight is seconds. Stop procrastinating with our study reminders. Posted 3 years ago. And at different points of the detector, you will detect each of these isotopes. The selected candidate will perform western blots, immunoprecipitation, protein purification, and prepare samples for mass spectrometry analysis. Well explore how you calculate this figure in just a second, but first lets look at how we can get data from TOF spectrometry.
The separated ions are then measured, and the results displayed on a chart. And that stems from the idea These represent isotopes that have relative abundances of 90% and 10% respectively. And because they have charge, they can be accelerated But in introductory chemistry class, most of the time you will get things in terms of just straight-up atomic mass. So even if most of the electrons we fire from the electron source miss the target, enough are making contact for us to be able to measure it in MS. when you says in nature does he mean in all of nature or just 'the nature of the sample'? The phase that you want depends on the ionization method you use. Direct link to Ryan W's post Because isotopes exist. TOF mass analysers separate ions based on the differences in velocities after acceleration to about 20 kV and subsequent different arrival times at the detector. Further examples of functional group influence on fragmentation are provided by a selection of compounds that may be examined by clicking the left button below. The precise isotopic composition of chlorine and bromine is: The presence of chlorine or bromine in a molecule or ion is easily detected by noticing the intensity ratios of ions differing by 2 Da. For example, the small m/z=99 Da peak in the spectrum of 4-methyl-3-pentene-2-one (above) is due to the presence of a single 13C atom in the molecular ion. Direct link to Richard's post In MS we ionize a sample , Posted 2 years ago. of an element are and their relative abundance? In a time of flight spectrometer, magnesium-25 ions, each with a mass of kg are accelerated toJ and their time of flight is seconds. Stop procrastinating with our study reminders. Posted 3 years ago. And at different points of the detector, you will detect each of these isotopes. The selected candidate will perform western blots, immunoprecipitation, protein purification, and prepare samples for mass spectrometry analysis. Well explore how you calculate this figure in just a second, but first lets look at how we can get data from TOF spectrometry.